Technology
Liposomes and nanoparticulate systems are a widely accepted formulation strategy to improve drug delivery and optimize therapeutic outcomes for many drugs, natural ingredients, pharmaceuticals, and extracts.
The Problem
Hard-to-absorb ingredients with health benefits and potential clinical indications are:
- Difficult to absorb in the digestive tract due to low solubility.
- Inaccurate dosing not ready for FDA regulation.
- Not viable in consumer products for mass distribution.
- Demonstrated to have liver toxicity when in oil.
Our Lipid Encapsulation Solution
Our proprietary, water-based delivery technology solves for poor bioavailability, solubility, shelf stability, and label claim transparency:
- Encapsulate ingredients into water-based formulas for greater bioavailability.
- Is proven to be shelf-stable for 12 months.
- Requires shaking or refrigeration necessary.
- Ensure supply chain protection and ‘use by’ dates for consumers.
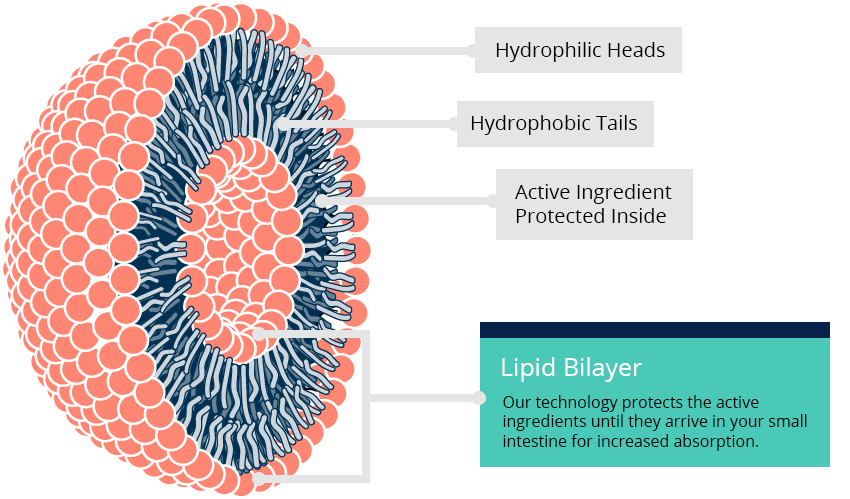
Liposomes are the key to the future of drug delivery technology.
Due to the presence of their lipid bilayer vesicle structure that closely resembles the make up of natural cells, liposomes are able to encapsulate drugs both within their aqueous core and their highly lipophilic membrane. As a result, the pharmacokinetics of drugs can be optimized when incorporated into a nanoparticle compared to drug alone providing meaningful delivery of difficult to formulate compounds.
Due to its versatility, biocompatible formulation attributes, and capacity to encapsulate highly lipophilic compounds, the utility of liposomes has experienced significant commercial, clinical and regulatory success as seen with FDA-approved drug products Doxil®, Myocet®, and AmBiosome®.
How the technology address compounds in biopharmaceutical classification system II
At Santé Laboratories, we’re focused on two fast-acting and bioavailable delivery platforms: aqueous liposomes and spray dried powders. We’re systematically expanding the application of the two platforms as well patents with subject matter expertise and experience.
Technology Advantages
- High solubility of water insoluble compounds
- Favorable physical and chemical stability
- Straight-forward Chemistry and Manufacturing Controls (CMC)
- High drug loading
- Rapid dissolution and predictable pharmacokinetics
- Highly reproducible particle size and narrow polydispersity
- Biocompatible, lipid-based excipients that are FDA and EU approved
- Scalable from benchtop to commercial (QbD and PAT eligible)
- Terminal sterilization by filtration
